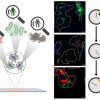
A research team from the Prodi Centre for Protein Diagnostics at Ruhr-Universität Bochum (RUB) has used infrared (IR) microscopes based on quantum cascade lasers to classify tissue samples of colorectal cancer from routine clinical operations in a marker-free and automated way. Artificial intelligence enabled the researchers to differentiate between different tumour types with great accuracy within approximately 30 minutes. Based on the classification, doctors can predict which course the disease will take and, consequently, choose the appropriate therapy. The team published their work in Scientific Reports.
A distinction is made between microsatellite stable (MSS) and microsatellite instable (MSI) tumours in colon and other cancers. Microsatellites are usually functionless, short DNA sequences that are frequently repeated. Patients with MSI tumours have a significantly higher survival rate. This is due to a mutation rate of cancer cells that is about 1000 times higher, which makes their growth less successful. Moreover, innovative immunotherapy is more successful in patients with MSI tumours. “It is therefore important for the prognosis and the therapy decision to know what kind of tumour we are dealing with”, says Professor Anke Reinacher-Schick, Head of the Department of Haematology and Oncology at the RUB clinic St Josef Hospital. To date, differential diagnosis has been carried out by immunohistochemical staining of tissue samples with subsequent complex genetic analysis.
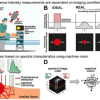
The potential of IR imaging as a diagnostic tool for the classification of tissue (so-called label-free digital pathology) has already been demonstrated in earlier studies by the group headed by Professor Klaus Gerwert from the RUB Department of Biophysics. The method recognises cancer tissue without prior staining or other marking and, consequently, also works automatically with the aid of artificial intelligence. Unlike the conventional differential diagnosis of microsatellite status, which takes about one day, the new method requires only about half an hour.
The protein research team has significantly improved the method by optimising it for the detection of a molecular change in the tissue. Previously, the tissue could be only morphologically visualised. “This is a big step that shows that IR imaging can become a promising method in future diagnostics and therapy prediction”, says Klaus Gerwert.
In collaboration with the Institute of Pathology at RUB headed by Professor Andrea Tannapfel and the Department of Haematology and Oncology at the RUB St Josef Hospital, the research team conducted a feasibility study with 100 patients. It showed a sensitivity of 100 % and a specificity of 93 %: all MSI tumours were correctly classified with the new method, only a few samples were falsely identified as MSI tumours. An expanded clinical trial is now starting, which will be carried out on samples from the Colopredict Plus 2.0 registry study. Initiated by Andrea Tannapfel and Anke Reinacher-Schick, the registry study allows the validation of the results from the published work. “The methodology is also of great interest to us, because very little sample material is used, which can be a decisive advantage in today’s diagnostics with an increasing number of applicable techniques”, explains Andrea Tannapfel.
In future, the method is to be introduced into the clinical workflow to assess its potential for precision oncology. “Following an increasingly targeted therapy of oncological diseases, it is very important to provide rapid and precise diagnostics”, concludes Anke Reinacher-Schick.