John Hammond
Starna Scientific Ltd, 52–54 Fowler Road, Hainault, Essex IG6 3UT, UK
In the first of our new Quality Matters columns (Issue 20/1), I introduced the new team of contributors and mentioned that John Hammond would be writing, from his position as the UK Industrial Delegate to the ISO-REMCO Committee, about the workings of that important body and looking to the future following some important decisions taken at the June 2007 meeting in Japan. This is his first of what will be a regular series of reports on the workings of REMCO.
Peter Jenks
The ISO Committee on Reference Materials (ISO-REMCO) was formally established in 1975, following a series of international symposia and meeting from May 1969 on.1 Its aim covers the normalisation of all aspects of reference materials (RMs), including establishment of definitions, categories, levels and classification of RMs.
The use of certified reference materials (CRMs) and reference materials (RMs) provides one of the “foundation stones” for quality assurance in both measurement and calibration laboratories. Used correctly, they provide a direct route to method validation and verification of assigned measurement values.
Reference materials, by definition, may be used for: the calibration of an apparatus, for the assessment of measurement methods, for establishing traceability of the measurement results or for determining the measurement uncertainty associated with measurement results. All these fields of RM application are integral laboratory activities and as such are included in ISO/IEC 17025—the most widely used standard for laboratory accreditation.
For this reason, i.e. their use in regulated environments, RM users expect their RM producers to demonstrate a similar level of quality, with respect to the production and metrological properties of their products. This is increasingly done via accreditation according to international guidance on requirements for the competence of reference material producers (ISO Guide 34 and ILAC G12), in conjunction with ISO/IEC 17025.
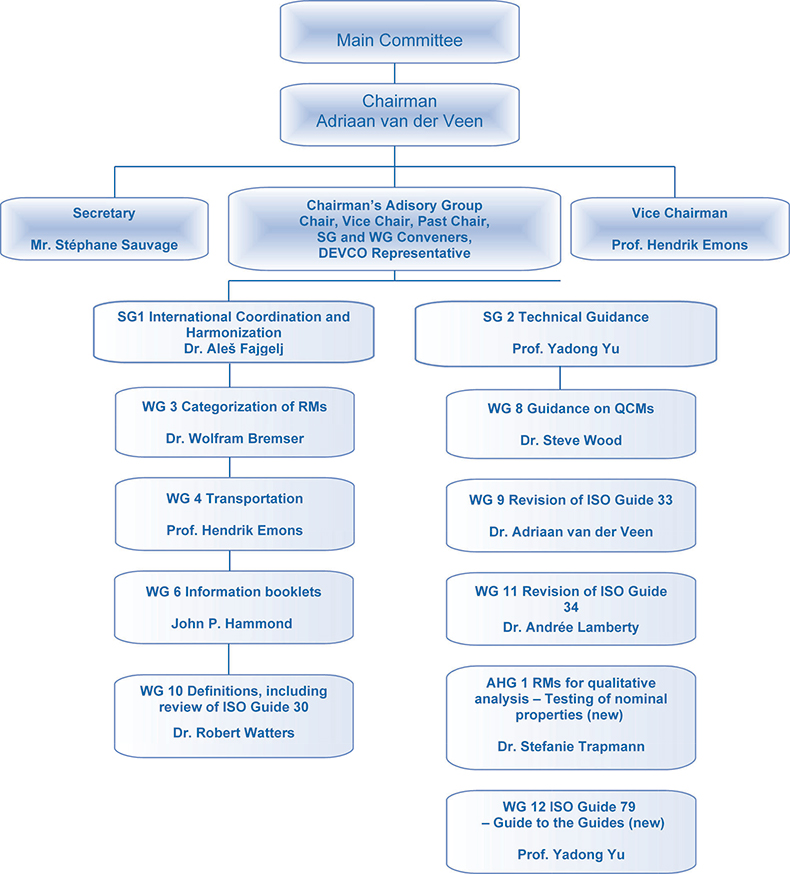
Activities performed by REMCO, as described in its initial terms of reference, include the establishment of definitions, categories, levels and classification of RMs; determination of structure of related forms of RMs; formulation of criteria to be applied for the choice of sources for mentioning in ISO documents; preparation of guidelines for ISO technical committees for making references to RMs in ISO documents; proposing actions to be taken on RMs required for ISO work; and dealing with matters within its competence arising in relation with other international organisations and to advise the ISO Council on these actions.
Following this mandate, REMCO has evolved into an important international body. REMCO membership in July 2007 included 69 ISO members, comprising 31 participating members and 38 observer members, with 19 international organisations in liaison. Major stakeholders are standardisation bodies, metrology institutions and international and regional liaison partners. REMCO clients—beside the ISO internal committees to which the REMCO has an advisory function—are producers and users of reference materials as well as accreditation bodies.
The ISO REMCO work is best reflected through ISO Guides on reference materials. The following guides have been prepared so far:
- ISO Guide 30: 1992 (Second edition)
Terms and definitions used in connection with reference materials—currently under revision; - ISO Guide 31: 2000 (Second edition)
Reference materials—Contents of certificates and labels—systematic review of this guide has recently been done, possible revision pending; - ISO Guide 32: 1997 (First edition)
Calibration of chemical analyses and use of certified reference materials—(no further action foreseen); - ISO Guide 33: 2000 (Second edition)
Uses of certified reference material production—currently under revision; - ISO Guide 34: 2000 (Second edition)
General Requirements for the Competence of reference materials producers—currently under revision; - ISO Guide 35: 2006 (Third edition)
Certification of reference materials—General and statistical principles.
In the last two years, REMCO has worked on the production of a guidance document for the manufacture and use of reference materials at the lowest level of a metrological quality assurance “pyramid”. Whilst these materials have a variety of names, e.g. QC material, precision control reference etc., their use is principally to provide basic “evidence of control” for a given system. Currently in draft format, this new Guide 80 will be discussed at the meeting in Brazil in June 2008.
At the last REMCO annual meeting in June 2007 in Tsukuba, Japan, study items relating to CRMs for qualitative analysis (testing of nominal properties) were initiated. This GAP analysis has confirmed that the testing of nominal properties has so far not been treated as a metrological topic and there is a general lack of relevant guidance in this area, including guidance related to reference materials. Appropriate CRMs have to serve the quality assurance needs for such testing. For some applications, such as in the identification of drugs, RMs already exist. The problem of estimating uncertainties for results of qualitative analysis has still to be solved in many cases.
Although the number of ISO Guides related to reference materials is not large, these guides cover very different areas of measurement, from production to the practical use of RMs. To improve the use and understanding of these ISO Guides, a new work item, development of an introductory Guide (ISO Guide 79), was initiated in 2007.
One of the key topics covered by REMCO has been terminology related to reference materials. The need to elaborate and define the most important terms of general use in the field of RMs was the basis for the origin of ISO Guide 30 Terms and definitions used in connection with reference materials. Consequently, REMCO definitions were adopted by all major standards and related documents for a few decades. Scientific development in all measurement areas, however, required definitions of RM and CRM to be reviewed and updated. This adoption happened in 2005 at the REMCO annual meeting in Geneva. The definitions are:
Reference material (RM)
Material, sufficiently homogeneous and stable with respect to one or more specified properties, which has been established to be fit for its intended use in a measurement process.
Note 1: RM is a generic term.
Note 2: Properties can be quantitative or qualitative, e.g. identity of substances or species.
Note 3: Uses may include the calibration of a measurement system, assessment of a measurement procedure, assigning values to other materials, and quality control.
Note 4: An RM can only be used for a single purpose in a given measurement.
Certified reference material (CRM)
Reference material, characterised by a metrologically valid procedure for one or more specified properties, accompanied by a certificate that provides the value of the specified property, its associated uncertainty and a statement of metrological traceability.
Note 1: The concept of value includes qualitative attributes such as identity or sequence. Uncertainties for such attributes may be expressed as probabilities.
Note 2: Metrologically valid procedures for the production and certification of reference materials are given in, among others, ISO Guides 34 and 35.
Note 3: ISO Guide 31 gives guidance on the contents of certificates.
The reader would realise that in the new definition RM is considered as a generic term. In the definition of CRM more emphasis has been placed on metrological characteristics of these materials than before, at the same time understanding that reference materials are used in quantitative measurements as well as in qualitative testing. Unfortunately, these new REMCO definitions were not accepted for the third edition International Vocabulary of Metrology—Basic and General Concepts and Associated Terms (VIM). The International measurement community will therefore be confronted with two different sets of definitions.
For many years REMCO concerned itself with the problems related to the dispatch of reference materials. Although the global acceptance of general custom tariff number (3322.00) for CRM was REMCO’s success, significant cross-border transport problems still remain.
REMCO has established an extensive liaison with relevant international bodies. The long lasting cooperation between REMCO and ILAC is a good example of common interest in effective and efficient preparation and utilisation of reference materials. REMCO continues its close work with other international organisations such as BIPM, ILAC, European Pharmacopoeia, US Pharmacopoeia, WHO and the World Anti-Doping Agency with the view to addressing and solving those problem.
Reference
- H.F. Steger, Accred. Qual. Assur. 7, 134–145 (2002). https://doi.org/10.1007/s00769-002-0451-5