Christoph Krafft,a Allison Stelling,b Jürgen Poppa,c and Reiner Salzerb
aInstitute of Photonic Technology, Albert-Einstein-Str. 9, 07745 Jena, Germany
bInstitute of Bioanalytical Chemistry, Dresden University of Technology, 01062 Dresden, Germany
cInstitute of Physical Chemistry, Helmholtzweg 4, 07743 Jena, Germany
Introduction
Interest in using Raman spectroscopy as a bioanalytical technique that can be applied to clinical problems continues to increase. Raman spectroscopy probes molecular vibrations and is based on inelastic scattering of monochromatic light. The inherent vibrations of samples provide a specific fingerprint without labels, in a non-destructive way and with diffraction limited resolution, which can be in the sub-micrometre range. A recent review summarised the wide field applications of Raman and coherent anti-Stokes Raman scattering (CARS) spectroscopy to characterise cells and tissue that are expected to gain significance in the future, such as the combination with imaging, microscopy and fibre optic probes.1
The most frequent applications include Raman spectroscopy of bone, skin, epithelial and muscular tissue, nervous tissue and tissues of the lung. The main scopes are the detection of pathologies and the recognition of diseases. Here, we focus on new trends in Raman spectroscopy to improve in vivo diagnosis. The use of Raman spectroscopy for real-time diagnosis of medical disease without the need for biopsy is among the most exciting and clinically relevant applications; four recent reports are presented. First, an approach to reduce fluorescent background of lung tissue in combination with a biomedical filtered Raman fibre optic probe was introduced in 2009 by Magee et al.2 Second, a fibre optic probe was developed for the CARS variant of Raman spectroscopy.3 Third, functional metal nanoparticles and carbon nanotubes were applied to a small animal model to collect Raman spectra non-invasively utilising the surface enhanced Raman scattering (SERS) effect.4 Finally, spatially offset Raman spectroscopy (SORS) has been presented as another non-invasive Raman-based method to probe deep bone subcutaneously in an animal model.5
Discussion
Biomedical Raman fibre optic probes
Biomedical Raman fibre optic probes are an essential component for the in vivo use of Raman spectroscopy. However, several challenges remain: (i) Raman scattering of tissue is inherently a weak phenomenon, (ii) Raman scattering is generated as the excitation light passes through the fibre optics and (iii) tissue generally exhibits high background fluorescence, even with the frequently used 785 nm excitation lasers. These challenges must be met while maintaining a safe level of laser exposure to the patient. Besides efficient collection of Raman signals the key requirements of a biomedical probe are: a compact size (2.5 mm outer diameter at maximum) and flexibility so it can be inserted into the working channel of standard endoscopes, for example, to study relatively inaccessible organs such as the lung during bronchoscopy or the colon during colonoscopy.
Raman fibre optic probes are generally made of low-OH fused silica because of its low Raman activity. However, large background signals still appear in the 200–1300 cm–1 range, which can be suppressed by use of bandpass filters with the excitation fibres; elastically scattered Rayleigh light is excluded from the detection side by use of longpass filters with the collection fibres. In combination with a Raman fibre optic miniprobe from Emvision LCC (Florida, USA) an approach called shifted subtracted Raman spectroscopy (SSRS) was reported to reduce the fluorescence in lung tissue2 that swamps the much weaker biological Raman signal of interest. The authors used a specifically designed probe, which consisted of a 400 µm diameter excitation fibre surrounded by seven 300 µm diameter collection fibres. The filtered signals were then refocused onto a bundle of 100 µm diameter fibres that were then aligned into a slit of a custom built Raman spectrometer to maintain the Raman intensities while improving spectral resolution. SSRS involves obtaining a Raman spectrum of the sample, then shifting the spectrum by approximately 20 pixels along the detector and retaking the Raman spectrum at the same spatial position on the sample. Since the so-called fixed pattern response (FPR) from the detector and the sample fluorescence vary little between the two spectra, subtracting one spectrum from another virtually eliminates the FPR and fluorescence, leaving the characteristic Raman bands of the sample in the form of a derivative-like spectrum which can then be reconstructed to a normal Raman response. The technique was applied to specimens of seven patients undergoing resection for lung cancer. Normal and tumour spectra were successfully distinguished by multivariate chemometric analysis. Although the study was performed ex vivo the principle is expected to be transferred soon to in vivo situations.
CARS microscopy
Coherent anti-Stokes Raman scattering (CARS) microscopy enables label-free imaging at sub-µm resolution and at fast image acquisition rates. Among the advantages of the non-linear effect are strong enhancement of the Raman signals due to the coherent nature of the process, the absence of autofluorescence background and the directional emission.
Whereas the diagnostic capabilities of CARS microscopy are promising, the practical implementation of the technique for clinical studies is currently rather limited. To optimise this imaging technology for clinical studies and to utilise the CARS advantages in vivo, a fibre-based probe for maximum collection of the CARS signal in biological tissue was demonstrated.3 The design challenges include capturing the backscattered forward generated CARS signal in the sample and reducing the effects of fibre non-linearities on the propagating pulses. To spectrally suppress four-wave mixing contributions in the fibre, the probe design incorporated separate fibres for excitation light delivery and for signal detection in combination with dichroic optics. Furthermore, scanning optics and an objective lens was integrated. Different biological tissues were imaged ex vivo in order to assess the performance of the fibre-delivered probe for CARS imaging (see, for example, Figure 1). Subcutaneous fat (1a), individual adipocytes (1b) and meibocytes (1c) were resolved with high contrast. In the future, it will be required to miniaturise the design for use as a handheld probe.

SERS
Non-invasive deep tissue molecular images have been presented from a living mouse with the use of Raman spectroscopy and surface enhanced Raman scattering (SERS) nanoparticles.4 For the study, a Raman system was adapted for small animal imaging and SERS active nanoparticles from Oxonica (CA, USA), namely Nanoplex Biotags, were used.
The SERS effect is well established as a technique to overcome the limited sensitivity of the Raman effect. Here, molecules adsorbed onto nano-roughened noble metal surfaces experience a dramatic increase in the incident electromagnetic field resulting in high Raman intensities comparable to fluorescence. The approach holds significant potential as a strategy for biomedical imaging of living subjects. Compared to fluorescence or bioluminescence strategies and other non-optical modalities (positron emission tomography, single photon emission computed tomography, magnetic resonance imaging, computed tomography) SERS nanoparticles offer a high multiplexing capacity that means multiple targets can be interrogated simultaneously.
The near infrared excitation and emission profiles of the SERS nanoparticles were ideal for minimising light absorption by tissue. The glass coating of the SERS nanoparticles guaranteed physical robustness, insensitivity to environmental conditions and simple biofunctionalisation of the well studied silica surface chemistry. Quantitative analysis software calculated the concentration of SERS nanoparticles and generated an image showing their accumulation in the liver (see Figure 2). This is consistent with previous observations that nanoparticles of various types are taken up by the reticuloendothelial system and thus can be found in the Kupffer cells of the liver. SERS nanoparticles were visualised in the liver region out to 24 days after injection. The published article4 demonstrated that by using targeted SERS nanoparticles and non-invasive in vivo imaging Raman spectroscopy can become an important clinical diagnostic tool.

SORS fibre-optic probes
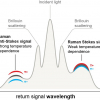
SORS was developed so that depth resolved sub-surface Raman measurements could be made in highly scattering materials such as tablets, polymers and animal tissue. In SORS, fibre-optic probes provide a spatial separation between the excitation laser illumination and Raman scatter collection regions. When the distance between the illumination and collection regions is increased, surface signals become less prominent and the collected Raman spectra contain a greater contribution from sub-surface components.
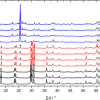
Pioneered by Pavel Matousek at the Rutherford Appleton Laboratory in the UK, SORS was recently applied to probe murine bone tissue transcutaneously by Raman spectroscopy.5 Although bone tissue was measured earlier by Raman spectroscopy in vivo, the new study presented a systematic approach to accurately recover in vivo bone Raman spectra from overlapping soft tissue in transcutaneous Raman measurements. The recovery of bone spectra through skin is complicated by fluorescence, light scattering and light absorption. The illumination–collection geometry was tailored to optimise Raman measurements for a particular bone size and depth. Figure 3 compares Raman spectra obtained from five fibre-optic probe configurations using the PhAT Raman fibre-optic probe accessory from Kaiser Optical Systems (MI, USA). The phosphate peak of the mineralised matrix hydroxyapatite at 960 cm–1 is shown at a magnified scale. The “line/disk 1” probe configuration was selected for in vivo Raman spectra of bone tissue in 32 live mice because it provided the best combination of sub-surface bone signal, signal variance and laser power distribution. Here, a cylinder lens was used to shape and focus a 5 × 1 mm laser line onto the specimen. The carbonate-to-phosphate ratios of bone tissue in the mouse tibia were determined from non-invasive Raman spectroscopic measurements and from measurements on the exposed bone tissue. Both values were found to be precise and accurate, that means neither the mean nor the standard deviation of the ratios varied significantly across five acquisitions.

Summary
The results obtained from in vivo Raman measurements, outlined briefly here, demonstrate that the method is suitable for clinical applications. However, before the technology can be transferred from use on animals to humans the possible thermal damage of the laser irradiation has to be excluded. In addition, further research is required to optimise the instrumentation and to make it user-friendly for a wider dissemination out of the optical laboratory into the clinical environment.
References
- C. Krafft, B. Dietzek and J. Popp, “Raman and CARS spectroscopy of cells and tissues”, Analyst 134, 1046–1057 (2009).
- N.D. Magee, J.S. Villaumie, E.T. Marple, M. Ennis, J.S. Elborn and J.J. McGarvey, “Ex vivo diagnosis of lung cancer using a Raman miniprobe”, J. Phys. Chem. B 113, 8137–8141 (2009).
- M. Balu, G. Liu, Z. Chen, B.J. Tromberg and E.O. Potma, “Fiber delivered probe for efficient CARS imaging of tissues”, Optics Express 18, 2380–2388 (2010).
- S. Keren, C. Zavaleta, Z. Cheng, A. de la Zerda, O. Gheysens and S.S. Gambhir, “Noninvasive molecular imaging of small living subjects using Raman spectroscopy”, Proc. Natl. Acad. Sci. 105, 5844–5849 (2008).
- M.V. Schulmerich, J.C. Cole, J.M. Kreidler, F. Esmonde-White, K.A. Dooley, S.A. Goldstein and M.D. Morris, “Transcutaneous Raman spectroscopy of murine bone in vivo”, Appl. Spectrosc. 63, 286–295 (2009).