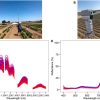
At this week’s BiOS, the biomedical optics and biophotonics exhibition at SPIE Photonics West, imec are presenting a promising path to the in vivo detection of low-grade gliomas (a group of slow-growing brain tumours). The breakthrough was realised by mounting imec’s snapscan VNIR 150 hyperspectral camera on a standard OR-approved surgical microscope. Intraoperative pilot tests were performed at the neurosurgical department of the University Hospital Leuven (KU Leuven). A milestone, imec researchers found the compact set-up to generate accurate clinical data, ready to be fed into a neural network that can convert those data into actable knowledge. In time, the approach could help surgeons detect intrinsic brain tumours’ exact demarcations intraoperatively and label-free, which would make for a whole new way of providing medical care.
Low-grade gliomas are a diverse group of (slow-growing) brain tumours that often arise in young, otherwise healthy patients. While typically considered benign in origin, studies have shown that low-grade gliomas can expand at a rate of 4–5 mm annually and come with the risk of malignant transformation. The tumour’s early surgical resection has thus become a much-favoured treatment option—although in vivo detection of low-grade gliomas and retrieving their exact demarcations is notoriously hard, even with the aid of surgical microscopes.
“Giving surgeons the proper tools to detect these tumours in vivo would make for an important breakthrough. Hyperspectral imaging (HSI) technology shows great potential to do just that”, says Roeland Vandebriel, field application engineer at imec. “Using HSI, brain tissue is illuminated, after which its reflected light is captured in many narrow spectral bands—resulting in a different spectral signature for healthy and anomalous cells.”
Bulky hardware and integration challenges have prevented HSI technology from being straightforwardly used in hospitals’ operating theatres. Yet, two presentations at this year’s BiOS now show a promising path forward.
Roeland Vandebriel: “It is a breakthrough we realised by mounting imec’s snapscan VNIR 150 hyperspectral camera on a surgical microscope. We found this compact set-up to generate accurate clinical data that can then be fed into a deep-learning neural network to convert the data into actable knowledge, allowing surgeons to discriminate between healthy and anomalous tissue. Clearly, these learnings are an important first step to accommodating the in vivo detection of intrinsic brain tumours—such as low-grade gliomas.”
Thanks to its small form factor (10 × 7 × 6.5 cm, and weighing 645 g), and its compatibility with standard C-mount optics, imec’s snapscan VNIR can easily be mounted on a surgical microscope. It makes for a compact set-up that can be incorporated in hospitals’ stringent clinical workflows, contrary to the bulky systems used in previous studies.
“Imec’s contribution also focused on how to acquire accurate and relevant clinical data in a (sterile) intraoperative environment”, comments Siri Luthman, imec’s project lead. “It had us rethink HSI’s existing spatial and spectral calibration methods, and necessitated us to interface imec’s snapscan directly with the surgical microscope’s optics and light source. As such, the first feasibility data set was generated—allowing us to evaluate HSI’s potential for the in vivo detection of low-grade gliomas; an approach coming with minimal adaptations to both the system and our data pre-processing methods.”
“Lighting is one of the other parameters we studied. To avoid excessive noise, we adapted the spectral range of the snapscan camera to match that of the microscope’s internal light source. We also looked at the system’s integration time modalities to find the right balance between saturation and noise. And last, we defined the microscope’s ideal zoom level and working distance to optimise the field of view”, she adds.
“Today’s findings indicate which hyperspectral bands are critical when interfacing with high-end surgical microscopes for an in vivo classification of low-grade gliomas. While intraoperative use of our set-up is premature, the approach has so far been validated using a clinical dataset of six patients at Belgium’s Leuven University Hospital. Going forward, we aim to further this project by integrating our snapshot technology, which accommodates video-rate hyperspectral imaging. This should allow us to explore the real-time detection of low-grade gliomas in surgical practice”, Roeland Vandebriel concludes.
This study was conducted in collaboration with Carl Zeiss Meditec AG. The results are presented at SPIE BiOS 2023 in two papers: Integrating Hyperspectral Imaging in an Existing Intra-Operative Environment for Detection of Intrinsic Brain Tumours, R. Vandebriel (imec) and Intra-Operative Brain Tumor Detection with Deep Learning-Optimized Hyperspectral Imaging, X. Zhang (Carl Zeiss Meditec AG).