Eric K.S. Shim and Soo-Y. Lee*
Division of Chemistry & Biological Chemistry, School of Physical & Mathematical Sciences, Nanyang Technological University, 21 Nanyang Link, Singapore 637371, Singapore. E-mail: sooying@ntu.edu.sg
Introduction
Edible bird’s nest (EBN)
Edible bird’s nest (EBN) is one the most costly food products of the Orient. EBN is especially valued by the Chinese as a quintessential food for its reputed health benefits, and has been documented in scientific publications since the last century.1 Cleaned EBN, retailing for a few thousand US dollars per kg, has sometimes been referred to as the “Caviar of the East”, but it has neither a relationship to, nor the appearance of fish eggs. The trade in EBN is estimated to be worth a few billion US dollars annually, with a large market, particularly in China. The swiftlet species that builds the EBN is native to South-East Asia, with Indonesia, Malaysia and Thailand the top three largest suppliers of EBN.
EBN is built strand by strand over a period of about a month using a viscous liquid secreted from a gland under the tongue of the swiftlet, primarily Aerodramus fuciphagus, during the breeding period. Unlike saliva, the secretion is for nest building and has no known digestive function. The bird interweaves feathers between the strands to form a strong composite material to hold the weight of a pair of nestlings and the parent birds. The nest is cleaned of feathers and other visible impurities before being sold in the market as cleaned EBN. Due to the high cost and driven by a profit motive, producers are often tempted to introduce edible adulterants into the cleaned bird nest.
EBN has been shown by proximate analysis to be a glycoprotein containing around 63% protein, 26% carbohydrate, 8% moisture, 2% ash/mineral and 1% lipid.2 The protein is made up of 17 types of amino acids: serine, valine, isoleucine, tyrosine, aspartic acid, asparagine, glutamic acid, glutamine, phenylalanine, arginine, glycine, threonine, alanine, lysine, histidine, leucine and methionine. The major carbohydrate saccharides are galactose, N-acetylneuraminic acid (Neu5Ac, a sialic acid), N-acetylgalactosamine (GalNAc), N-acetylglucosamine (GlcNAc), mannose and fucose.3
Adulteration of EBN
The common edible adulterants introduced into EBN can be classified into two types: Type I adulterants (e.g. tremella fungus, coralline seaweed, agar, fish bladder and pork rind) are water-insoluble with a similar external appearance to EBN and can be adhered to the surface of EBN strands; and Type II adulterants (e.g. sucrose, glucose, hydrolysed collagen and monosodium glutamate) are water soluble and can be absorbed within the EBN cement to form a uniform composite material on drying.4 The externally adhered Type I adulterants can be detected with a microscope and separated out for analysis, but not the Type II adulterants that have been incorporated into the EBN cement since the final product looks exactly like the unadulterated EBN.
Some laboratory techniques such as metabolite mapping,5 gel electrophoresis (GE)6 or enzyme-linked immunosorbent assay (ELISA),7 allow us to check for the authenticity of EBN, but they are slow, destructive, require bulk sample sizes and may require specialised personnel to perform the analysis.
Raman microspectroscopy
Raman spectroscopy is a simple, rapid, direct, requiring no sample preparation and non-destructive method to provide molecular information on a sample with good sensitivity and specificity. It is a light scattering technique that gives a molecular vibrational “fingerprint” of the chemical bonds present and thus allowing us to identify and quantify the chemicals in a sample. The laser beam and optical microscope allow focusing on a microscopic sample area of a few µm in diameter. The ease of use and the fact that it is not affected by the presence of water in a sample makes Raman microspectroscopy an increasingly important tool for characterisation and quality control in the food industry. It is an ideal tool for the study of EBN where moisture is present in the sample.
Materials and methods
EBN and adulterants
Raw, white EBN samples were obtained from bird houses in widely separated geographical locations in South-East Asia—West Malaysia, East Malaysia and Indonesia. All adulterants were purchased from commercial sources. In the preparation of samples with Type II adulterants, EBN was soaked in 2% to 10%, (w/w) aqueous solutions of sucrose, glucose, hydrolysed marine collagen or monosodium glutamate (MSG) overnight and air-dried to constant weight.
Raman microspectroscopy
Raman spectra were collected with the Ramantouch microspetrometer (Nanophoton Inc., Japan) under the same collection conditions (785 nm laser, LU Plan Fluor 20X, 600 gr/mm, 140 mW, 60 s) at 24°C. Every sample was measured in triplicate over different spots and all the Raman spectra were baseline corrected with Origin Pro 8.0 (OriginLab Corp., USA).
Results and discussion
Unique Raman spectrum of EBN
EBN made by the same species of swiftlets (Aerodramus fuciphagus) from different geographical locations show a unique Raman spectrum (Figure 1), where the Raman spectra are overlapped, indicating that they were made of the same material.
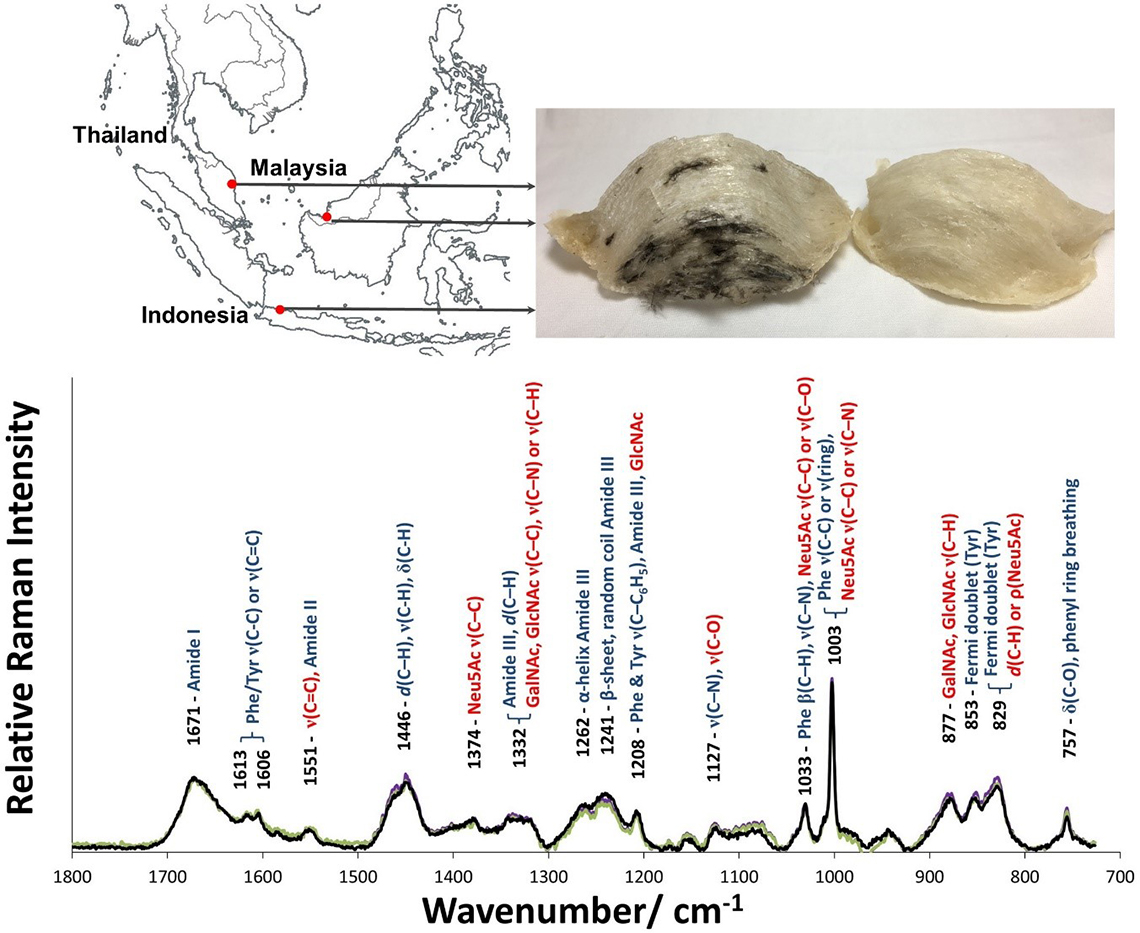
Figure 1. Top panel: Map of South-East Asia showing the geographical locations of Kuantan (West Malaysia), Kuching (East Malaysia) and Jakarta (Indonesia) where the EBN samples came from. Lower panel: the unique Raman spectra of EBN, shown overlapped, can be used as a standard for authentication. Peptides bands are shown in blue and saccharides in red, showing that EBN is a glycoprotein. (ν—stretching; α—out-of-plane bending; β—in-plane bending; δ—scissoring; ρ—rocking; d—deformation).
As EBN is a glycoprotein, Raman bands attributed to protein and carbohydrate can be observed and were assigned using spectral data of other glycoproteins,8N-acetylneuraminic acid,9 tyrosine10 and collagen11 as reference. The strong Raman bands that can be attributed to the protein component are: 1671 cm–1 (amide I), 1446 cm–1 (CH deformation) and 1241–1262 cm–1 (amide III). Various Raman bands of the saccharides, particularly sialic acid or N-acetylneuraminic acid (Neu5Ac), N-acetyl-glucosamine (GlcNAc) and N-acetyl-galactosamine (GalNAc) can be seen. Some of the bands for the saccharides overlap with those for the protein. In particular, the strong intensity of the 1003 cm–1 Raman line, with relative peak intensity about 2.2 times that of the amide I band, has contributions from both the ring vibration of phenylalanine (Phe) and the C–C and C–O stretches of sialic acid. From the Raman spectra of pork rind, fish bladder and hydrolysed marine collagen, which lack sialic acid, we deduce that phenylalanine contributes about the same intensity as the peak of the amide I band, and so about 55% of the intensity of the 1003 cm–1 Raman line in EBN is due to sialic acid. The unique Raman spectrum of EBN, using the band frequencies and the relative intensities of the bands, can be used as a standard for authentication.
Raman spectra of surface adhered Type I adulterants
Raman microspectroscopy allows measurements to be made over a microscopic area. Edible, surface adhered Type I adulterants (e.g. tremella fungus, agar, coralline seaweed, pork rind and fish bladder) can be picked up by a microscope as there are significant differences in their microscopic images as compared to EBN which is characterised by translucent strands of 1–2 mm thickness. The microscopic images of the Type I adulterants and EBN are shown in the right panel of Figure 2.
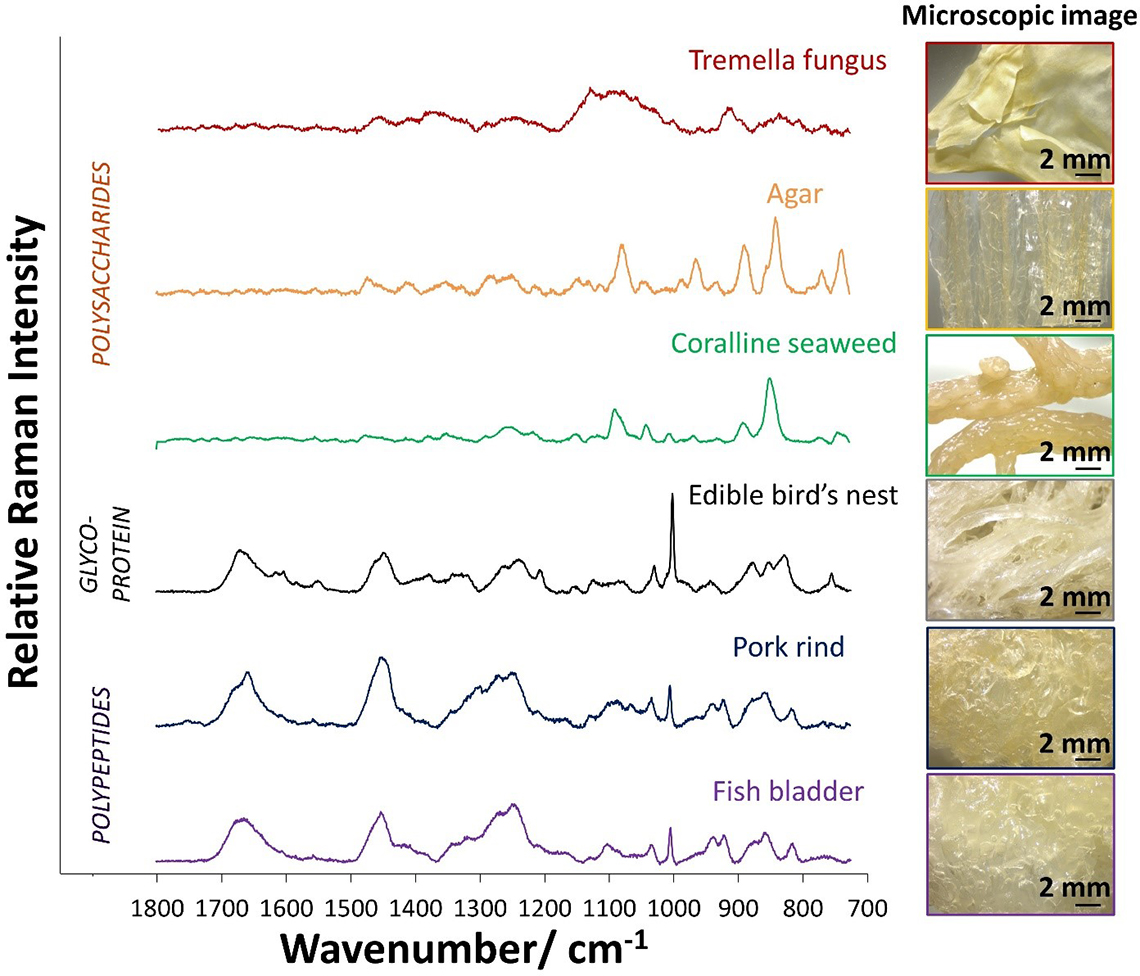
Figure 2. Left panel: Raman spectra of Type I adulterants, which may be polysaccharides or polypeptides, in comparison with EBN. Right panel: microscopic images of Type I adulterants and EBN.
The Raman spectra in the left panel of Figure 2 show that Raman spectroscopy can distinguish between the polysaccharides (e.g. tremella fungus, agar and coralline seaweed) which lack amide bands, the polypeptides (e.g. pork rind and fish bladder) with strong amide bands and EBN, a glycoprotein, with amide and saccharide bands. EBN has a particularly strong 1003 cm–1 Raman line due to phenylalanine and sialic acid, and a strong 830 cm–1 line due to sialic acid. Pork rind and fish bladder do not have sialic acid, and the weaker 1003 cm–1 Raman line is due to phenylalanine only; they also do not have the 830 cm–1 line. Instead, they show a medium intensity line at 810 cm–1 which is due to C–O–C stretching from lipids.
Raman spectra of composites of EBN with Type II adulterants
EBN can soak up an aqueous solution of edible Type II adulterants [e.g. sucrose, glucose, (hydrolysed marine) collagen and MSG] which upon drying gives a composite material that looks like unadulterated EBN. Any substance that is water soluble, forming a clear solution, can be a Type II adulterant to form a composite with EBN. The uptake of adulterants in the dried composite EBN as a function of the concentration of adulterant solutions used are shown in Figure 3, and we have used a quadratic least square fit of the data, but the plots are almost linear. The uptake of Type II adulterants in the dried composite EBN can be considerable. At 10% w/w of adulterant solutions, the uptake was about 40% w/w of sucrose, about 34% w/w of glucose, about 29% w/w of collagen and about 20% w/w of MSG.
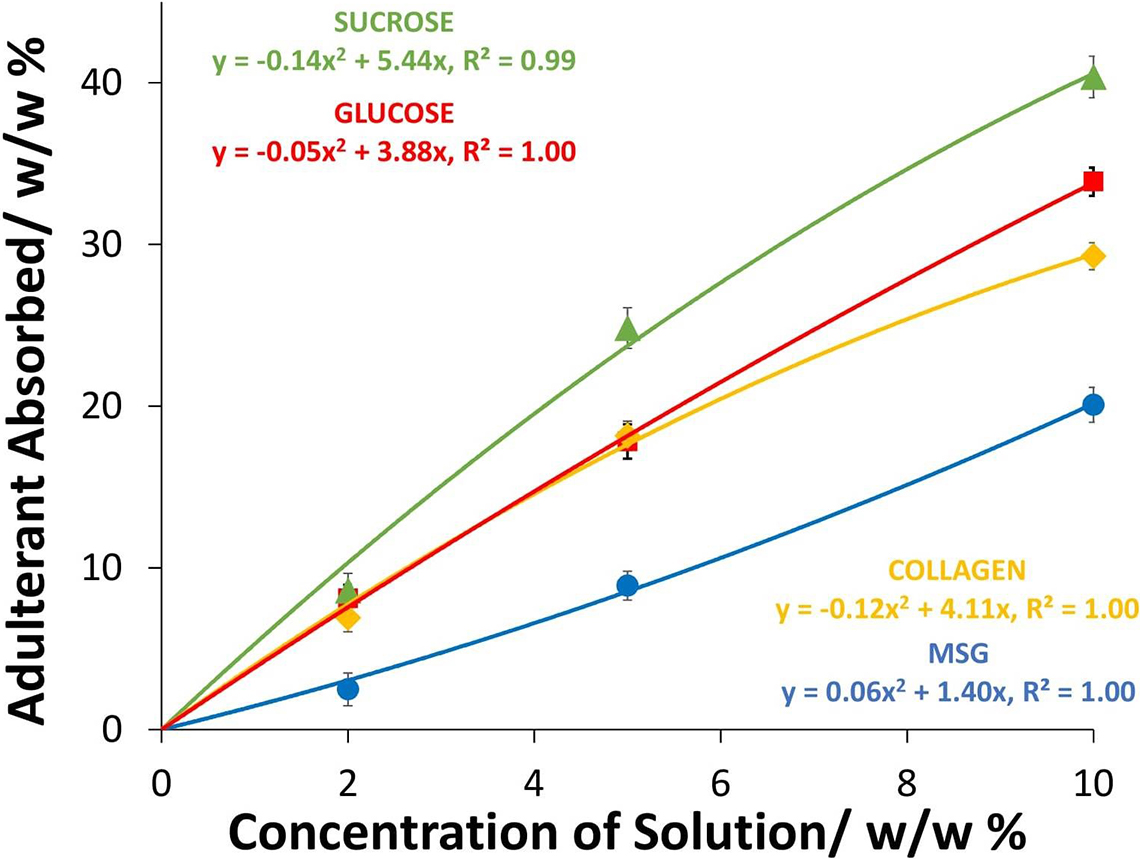
Figure 3. Graph of the uptake of the Type II adulterants by EBN when dried versus concentration of adulterant solutions used in soaking.
Raman microspectroscopy can detect the Type II adulteration of EBN. In Figure 4, we show the Raman spectra of the EBN composites with sucrose, glucose, collagen and MSG, which were prepared by soaking in 5% w/w of adulterant solutions, in comparison with unadulterated EBN.
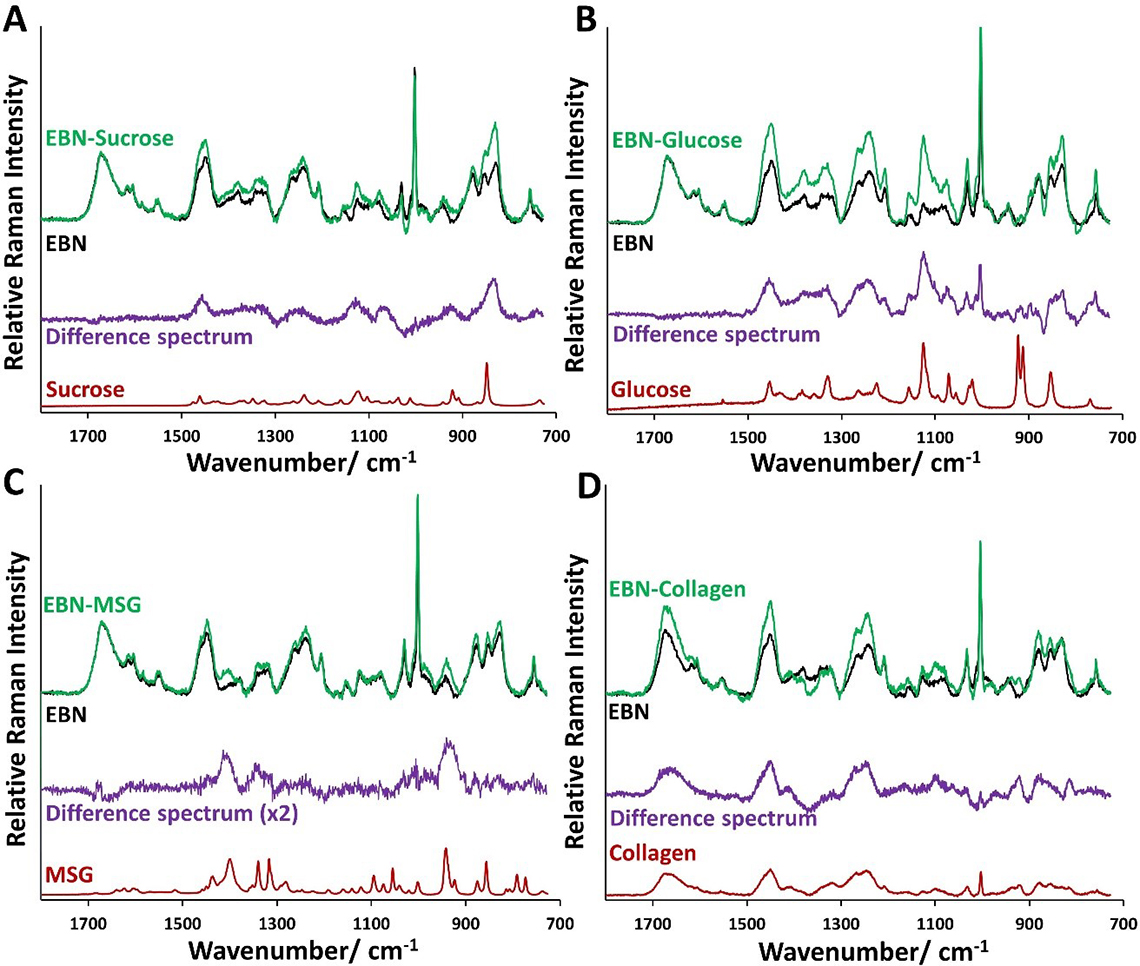
Figure 4. Comparison of normalised Raman spectrum of EBN composites with Type II adulterants (in green)—sucrose, glucose, (hydrolysed marine) collagen and MSG—prepared by soaking in 5% (w/w) solution of Type II adulterants with the Raman spectrum of unadulterated EBN (in black). The difference Raman spectra (in purple) resemble that of the adulterants (in red).
For sucrose, glucose and MSG which lack protein bands, the Raman spectra of the composites were normalised with respect to the amide I band of EBN, and the difference spectrum was calculated and compared with the corresponding Type II adulterant in each subplot in Figure 4 (A–C). For collagen which has Raman amide bands but no sialic acid, we have used the following information to scale the Raman intensity of the composite relative to EBN: (a) the composite has about 18% of collagen and about 82% of EBN as shown in Figure 3, (b) the intensity of the 1003 cm–1 Raman line is due to phenylalanine and sialic acid, with sialic acid coming solely from EBN, (c) the intensity of the 1003 cm–1 Raman line of the composite due to phenylalanine is the same as the peak intensity of the amide I band of the composite, and the rest of the intensity of the Raman line can be attributed to sialic acid. So, with EBN as the standard, we know the intensity of the sialic acid contribution at 100% EBN, and so we scale the Raman spectrum of the composite so that the intensity of the sialic acid component is 82% of that. The results are shown in Figure 4(D), together with the difference Raman spectrum and the Raman spectrum of collagen. It can be seen in Figure 4 that the difference Raman spectra resemble those of the corresponding adulterants, but the Raman lines in the difference spectra are generally broader for sucrose, glucose and MSG. The reason is because the Raman spectra of these adulterants were taken in the pure crystalline form, whereas in the EBN composites these molecules are embedded in an amorphous EBN cement. Collagen, however, is already in an amorphous form and so the difference spectrum has broad lines similar to that for collagen.
Conclusions
The investigation showed that EBN has a unique Raman spectrum that can be used as a standard for authentication. Raman microspectroscopy can distinguish between polysaccharides (no amide bands), polypeptides (strong amide bands) and glycoproteins (both amide and saccharide bands), and so can be used to detect edible, water-insoluble Type I adulterants which are polysaccharides or polypeptides and can be adhered to the surface of EBN strands. Clear, edible, water-soluble Type II adulterants can be adsorbed by EBN to form a composite that looks like the unadulterated EBN under a microscope. However, the EBN composites with Type II adulterants give rise to Raman spectra that differ from the unique Raman spectrum of unadulterated EBN. Raman microspectroscopy thus offers a rapid, non-destructive technique, requiring very little sample and no prior treatment to authenticate EBN.
References
- R.S.Y. Wong, “Edible bird’s nest: food or medicine?”, Chin. J. Integr. Med. 19(9), 643–649 (2013). https://doi.org/10.1007/s11655-013-1563-y
- M.F. Marcone, “Characterization of the edible bird’s nest the ‘Caviar of the East’”, Food Res. Int. 38(10), 1125–1134 (2005). https://doi.org/10.1016/j.foodres.2005.02.008
- F. Ma and D. Liu, “Sketch of the edible bird’s nest and its important bioactivities”, Food Res. Int. 48(2), 559–567 (2012). https://doi.org/10.1016/j.foodres.2012.06.001
- E.K.S. Shim, G.F. Chandra, S. Pedireddy and S.-Y. Lee, “Characterization of swiftlet edible bird nest, a mucin glycoprotein, and its adulterants by Raman microspectroscopy”, J. Food Sci. Tech. 53(9), 3602–3608 (2016). https://doi.org/10.1007/s13197-016-2344-3
- Y.G. Chua, S.H. Chan, B.C. Bloodworth, S.F.Y. Li and L.P. Leong, “Identification of edible bird’s nest with amino acid and monosaccharide analysis”, J. Agric. Food Chem. 63, 279–289 (2015). https://doi.org/10.1021/jf503157n
- L.T. Hun, W.A. Wani, H.Y. Poh, U. Baig, T.T.E. Tan, N.I. Nashiruddin, Y.E. Ling and R.A. Aziz, “Gel electrophoretic and liquid chromatographic methods for the identification and authentication of cave and house edible bird’s nests from common adulterants”, Anal. Methods 8, 526–536 (2016). https://doi.org/10.1039/C5AY02170G
- S. Zhang, X. Lai, X. Liu, Y. Li, B. Li, X. Huang, Q. Zhang, W. Chen, L. Lin and G. Yang, “Development of monoclonal antibodies and quantitative sandwich enzyme linked immunosorbent assay for the characteristic sialoglycoprotein of edible bird’s nest”, J. Immunoassay Immunochem. 34, 49–60 (2013). https://doi.org/10.1080/15321819.2012.680527
- L. Ashton, P.D.A. Pudney, E.W. Blanch and G.E. Yakubov, “Understanding glycoprotein behaviours using Raman and Raman optical activity spectroscopies: characterising the entanglement induced conformational changes in oligosaccharide chains of mucin”, Adv. Colloid Interface Sci. 199–200, 66–77 (2013). https://doi.org/10.1016/j.cis.2013.06.005
- E. Vinogradova, A. Tlahuice-Flores, J.J. Velazquez-Salazar, E. Larios-Rodriguez and M. Jose-Yacaman, “Surface-enhanced Raman scattering of N-acetylneuraminic acid on silver nanoparticle surface”, J. Raman Spectrosc. 45(9), 730–735 (2014). https://doi.org/10.1002/jrs.4544
- B. Hernández, Y.-M. Coïc, F. Pflüger, S.G. Kruglik and M. Ghomi, “All characteristic Raman markers of tyrosine and tyrosinate originate from phenol ring fundamental vibrations”, J. Raman Spectrosc. 47(2), 210–220 (2016). https://doi.org/10.1002/jrs.4776
- T.T. Nguyen, C. Gobinet, J. Feru, S.B. Pasco, M. Manfait and O. Piot, “Characterization of type I and IV collagens by Raman microspectroscopy: identification of spectral markers of the dermo-epidermal junction”, Spectrosc. Int. J. 27(5–6), 421–427 (2012). https://doi.org/10.1155/2012/686183